The timely and proper processing of clinical data is critical to the success of any scientific or medical endeavor. However, clinical data processing (CDP) is frequently disregarded or receives insufficient attention despite its significance. This may result in erroneous analysis and interpretation, which would ultimately affect the quality of the output. The processing of healthcare data gets more complicated every year. The amount and diversity of data that must be analyzed are always growing as new technologies and therapies are created. In order to guarantee patient safety, data integrity, and regulatory compliance, clinical research generates vast volumes of data that must be carefully monitored and handled. The adoption of cutting-edge technologies in clinical trials has made them more complex, making effective data management more important than ever. Data processing services aid in gathering, verifying, and preparing data for statistical analysis in clinical trials.
What Is Clinical Data Management?
Clinical data management (CDM) involves all aspects of clinical data processing, right from data collection and organization to making it available in structured and accessible format. It’s all about providing the right information to the right people at the right time. Advanced technologies and processes are available to collect, sort, organize, and evaluate clinical data. When it comes to clinical research, the process involves collecting, cleansing, and managing trial data, which is crucial to get reliable, statistically valid data from the clinical trial. Researchers need to acquire, store, and report on clinical research data in keeping with HIPAA regulations.
Building databases to collect, verify, clean, lock, code, and analyze data from sites is the responsibility of the data managers. This covers audit trails and other metadata in addition to patient data. Completeness, precision, validity, and consistency of the data are ensured by sound data management. It attests to the data’s dependability and standardization for statistical analysis that produces findings supported by evidence. As technology advances, remote data management and acquisition in clinical trials are made easier with centralized electronic data capture (EDC).
Why Clinical Research Needs Data Management
In a clinical research management system, credible data management is essential for the following main reasons:
- Ensures patient safety: Accurate data aids in the detection of safety concerns such as unfavorable trial occurrences. To better safeguard study participants, effective data management reduces data discrepancies and missing data.
- Delivers precise outcomes: The integrity of the test analysis is established by perfect data collection and management. Without it, errors may be made, defects may spread, and study findings may be called into doubt.
- Complies with regulations: Consistent quality control is ensured by protocol-mandated data monitoring and verification. This complies with all applicable laws, regulations, and standards, including 21 CFR 11 and HIPAA.
- Allows for immediate decisions: Quick query resolution is made possible by effective data management. This makes it possible to evaluate study milestones, recruiting, and subject retention using data-driven methods.
- Cuts expenses and time: EDC systems with good design encourage reliable data sharing. This reduces expenses associated with data inconsistencies and expedites research timelines.
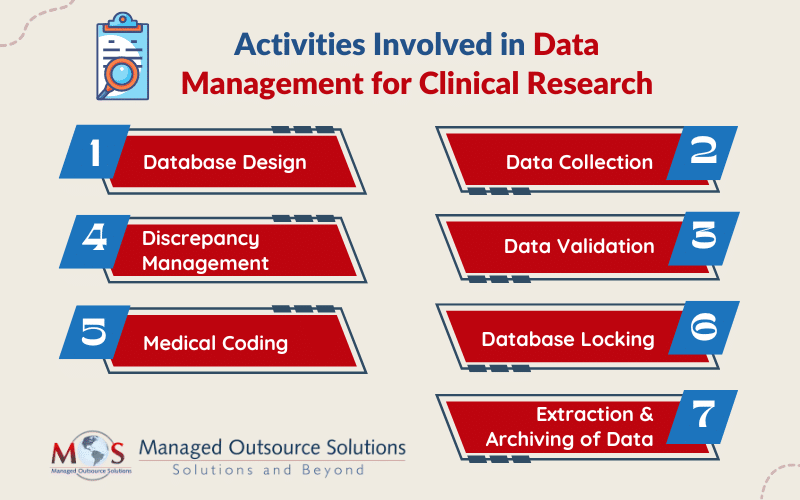
Different Activities involved in Data Management
The main activities involved in managing data for clinical research are:
-
- Database design: While designing a database, details like objectives, intervals, visits, investigators, sites, and patients are defined and tested.
- Data collection: Data capture can be accomplished with case report form (CRF), which is available either in paper or digital format. Data is manually entered into the system whereas data from digital forms can be used directly. Usually, double data entry is performed.
- Data validation: After the data is entered, it is verified for transcription errors and discrepancies. Discrepancies which may occur due to deviations from the protocol, missing data, inconsistent data, or range checks, are highlighted.
- Discrepancy management: Discrepancy management includes reviewing discrepancies, investigating its reasons, and resolving them with documentary proof. If the team can’t find any solution to the discrepancy, it will be declared as irresolvable.
- Medical coding: Coding (using MedDra, WHO-DDE for coding medications) for the clinical study is done as based on the project-specific protocol requirement.
- Database locking: When all the data management activities are complete, the database is locked and the cleansed and verified data is extracted for analysis.
- Extraction and archiving of data: The clean data is taken out of the database and subjected to statistical analysis to confirm its integrity and statistical significance after the database has been locked. High-level documents such as Investigator’s Brochures (IBs) and Clinical Study Reports (CSRs) integrate the results. After that, the information and required documentation are preserved to enable future study.
To understand the different methods of data processing
Read our blog post Five Different Data Processing MethodsTechnology breakthroughs have improved the quality and speed of data creation, which has been good news for clinical data management systems. The CDM processes are gradually moving from paper-based to electronic systems in order to meet the demands of regulatory bodies and pharmaceutical corporations for producing high-quality data for trustworthy assessment of medications, biologics, and medical devices. Outsourcing data management to providers of data processing services helps to streamline the workforce, maintain the quality standards of processes and provide clean, accurate data in a usable format, in a timely manner.
Get in touch to find out how our data processing services can improve your workflow!
-